Test result allows for urogenital tract (UGT) microbiota assessment by determining:
- proportion of normal biota and opportunistic pathogens in total bacterial load (TBL)
- content of spp., Ureaplasma spp., Mycoplasma hominis and Candida spp.
- presence of obligate pathogens

Biomaterial
Different biomaterial types are accepted for analysis: scrape from penis balanus and urethra, urine, prostate fluid, ejaculate, biopsy sample of prostate tissue.
To prevent uncertain results of male urogenital tract microflora composition determination due to transient microflora, we recommend to abstain from unprotected sexual contacts for 3 days before biomaterial collection.
- Types of biomaterial recommended for etiological diagnostics and monitoring of urethritis and balanoposthitis: scrape from urethra, scrape from foreskin of penis balanus, first portion of morning urine (only for pathogen identification).
- Types of biomaterial recommended for etiological diagnostics and monitoring of epididymitis, prostatitis, asymptomatic STIs: ejaculate, prostate fluid*, residual urine after prostate massage, biopsy sample of prostate tissue. Please note that prostate massage is strictly forbidden if acute prostatitis is suspected.


Indications
Urogenital tract microbiota state seriously affects male reproductive functions and quality of life. Microbiota imbalance may result in such complications as balanoposthitis, vesiculitis, epididymo-orchitis, prostatitis with further secondary infertility development etc.

Indications for the assay are:
-
Urethral discharge
-
Urinary disorders
-
Urethral discomfort
-
Redness, itching, swelling, rash
-
Unpleasant odor
-
Reproductive dysfunction
-
IVF preparation
-
Evaluation of therapy effectiveness and treatment outcomes
Androflor®test
Androflor® assay is performed by real-time PCR and allows for quick and high-precision determination of qualitative as well as quantitative microbiota composition based of microorganisms’ DNA fragments.
Unlike microscopy, PCR is an instrumental methodology, thus the risk of incorrect or subjective result evaluation is eliminated. Unlike bacteriological methods, PCR does not require maintaining microorganism viability before the biological sample reaches the laboratory and enables the detection of anaerobic microorganisms.
Androflor® Screen and Androflor®comparison
There are two available versions of the test:
- Androflor® Screen is designed for screening and differential diagnostics of acute forms of urogenital tract diseases.
- Androflor® is designed for diagnostics and monitoring of acute infectious and inflammatory diseases of male urogenital system.
| Indicators | Androflor® Screen | Androflor® |
|---|---|---|
| Total bacterial load (TBL) | ||
| Human genomic DNA (HGD) | ||
| Transient microflora: Lactobacillus spp. | ||
| Normal flora | ||
| Staphylococcus spp. | ||
| Streptococcus spp. | ||
| Corynebacterium spp. | ||
| Total: Normal flora | ||
| Bacterial vaginosis (BV) associated opportunistic microorganisms (OM) | ||
| Gardnerella vaginalis | ||
| Ureaplasma urealyticum | ||
| Ureaplasma parvum | ||
| Mycoplasma hominis | ||
| Atopobium cluster | ||
| Megasphaera spp. / Veillonella spp. / Dialister spp. | ||
| Sneathia spp. / Leptotrichia spp. / Fusobacterium spp. | ||
| Total: BV-associated OM | ||
| OM anaerobes | ||
| Bacteroides spp. / Porphyromonas spp. / Prevotella spp. | ||
| Anaerococcus spp. | ||
| Eubacterium spp. | ||
| Peptostreptococcus spp. / Parvimonas spp. | ||
| Total: OM anaerobes | ||
| OM: Preudomonas aeruginosa / Ralstonia spp. / Burkholderia spp. | ||
| OM: Haemophilus spp. | ||
| OM: Enterobacteriaceae spp. / Enterococcus spp. | ||
| Pathogens | ||
| Neisseria gonorrhoeae | ||
| Chlamydia trachomatis | ||
| Mycoplasma genitalium | ||
| Trichomonas vaginalis | ||
Report
Androflor®
Please note the color markers near numerical values in the table.
- Moderate deviation from the norm
- Significant deviation from the norm
*Absolute Lg(X) analysis
**Qualitative analysis
***Lower than threshold value
| № | Name of the test | Result | |
|---|---|---|---|
| Quantitative |
Relative (Lg(X/CBMO) |
||
| Human genomic DNA |
104.5
|
||
| 1 | Total bacterial load |
106.4
|
|
| Transient microflora | |||
| 2 | Lactobacillus spp. |
103.2
|
-3,2 (< 0,1%)
|
| Normal flora | |||
| 3 | Staphylococcus spp |
103.9
|
-2,5 (0,3-04%)
|
| 4 | Streptococcus spp |
103.5
|
-2,9 (0,1-0,1%)
|
| 5 | Corynebacterium spp |
104.5
|
-1,9 (1,1-1,4%)
|
| Total: Normal flora |
104.6
|
-1,8 (1,4-2,0%)
|
|
| BV-associated OM | |||
| 6 | Gardnerella vaginalis | Not detected |
|
| 7 | Megasphaera spp./Veilbnella spp./Dialister spp. |
10 5.2
|
-1,2 (5-7%)
|
| 8 | Sneathia spp./Leptotrichia spp./Fusobacterium spp. |
10 3.5
|
-2,9 (0,1-0,1%)
|
| 9 | Ureaplasma urealyticum* |
Not detected
|
|
| 10 | Ureaplasma parvum* |
Not detected
|
|
| 11 | Mycoplasma hominis* |
Not detected
|
|
| 12 | Atopobium cluster | Not detected |
|
| Total: bacterial vaginosis-associated OM | 10 5.2 |
-1,2 (5-7%)
|
|
| OM anaerobes | |||
| 13 | Bacteroides spp./Porphyromonas spp./Prevotella spp. | 10 6.1 |
-0,3 (43-58%)
|
| 14 | Anaerococcus spp. | 10 4.7 |
-1,7 (1,7-2,3%)
|
| 15 | Peptostreptococcus spp./Parvimonas spp. | 10 4.5 |
-1,9 (1,1-1,4%)
|
| 16 | Eubacterium spp. | 10 5.1 |
-1,3 (4-6%)
|
| Total: OM anaerobes | 10 6.2 |
-0,2 (50-67%)
|
|
| OM Haemophilus spp. | |||
| 17 | Haemophilus spp. |
Not detected
|
|
| OM Pseudomanas aeruginosa/Raistonia spp./Burkholderia spp. | |||
| 18 | Pseudomanas aeruginosa/Raistonia spp./Burkholderia spp. |
Not detected
|
|
| OM Enterobacteriaceae spp./Enterococcus spp. | |||
| 19 | Enterobacteriaceae spp./Enterococcus spp. | 10 5.9 |
-0,5 (27-36%)
|
| Yeast fungi | |||
| 20 | Candida spp* |
Lower than TV***
|
|
| Pathogens | |||
| 21 | Mycoplasma genitalium** |
DETECTED
|
|
| 22 | Trichomonas vaginallis** |
Not detected
|
|
| 23 | Neisseria gonorrhoeae** |
Not detected
|
|
| 24 | Chlamydia trachomatis** |
Not detected
|
|
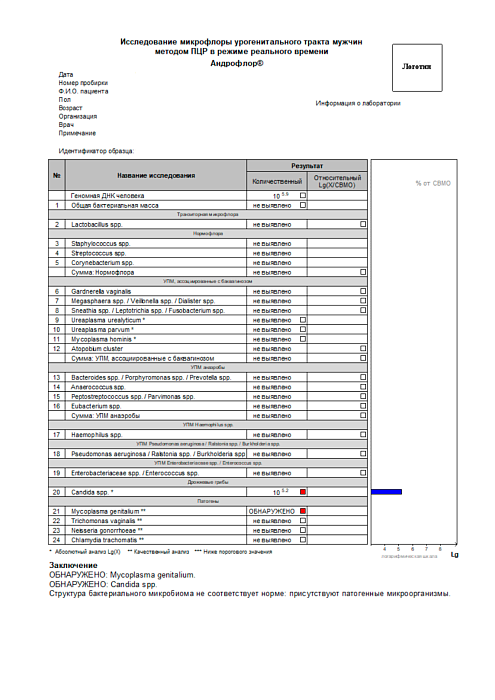
Conclusion
DETECTED: Mycoplasma genitalium.
Candida spp. is lower than threshold value.
Abnormal structure of bacterial microbiome: pathogens are detected, normal and opportunistic microflora balance is disrupted.
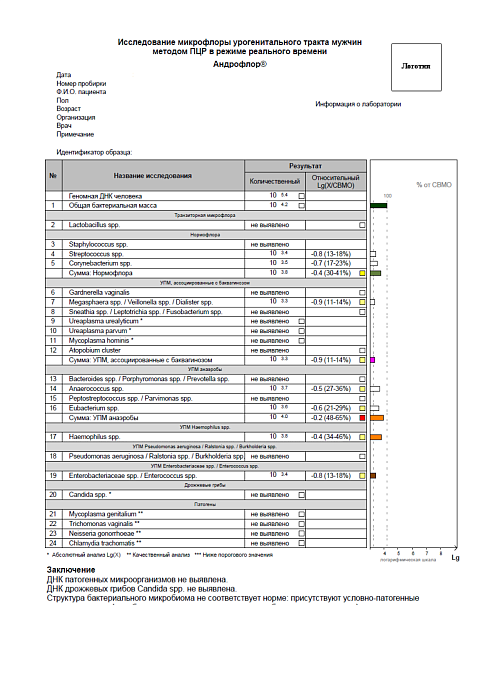
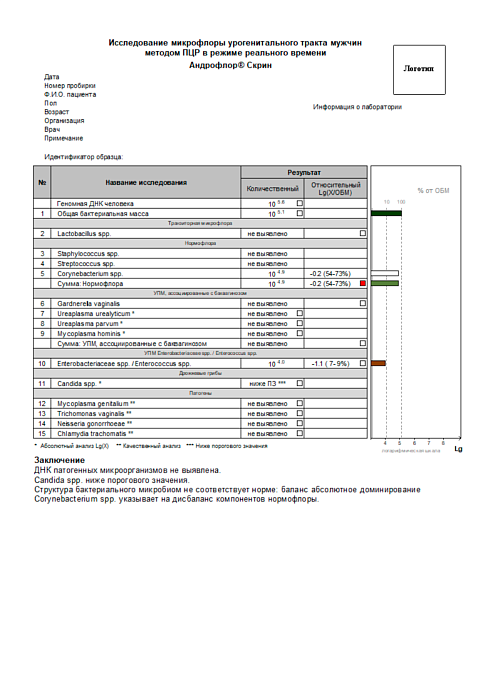
Report examples
F.A.Q.
-
Is there any special preparation needed for the assay? What should I know to collect biomaterial for Androflor®?
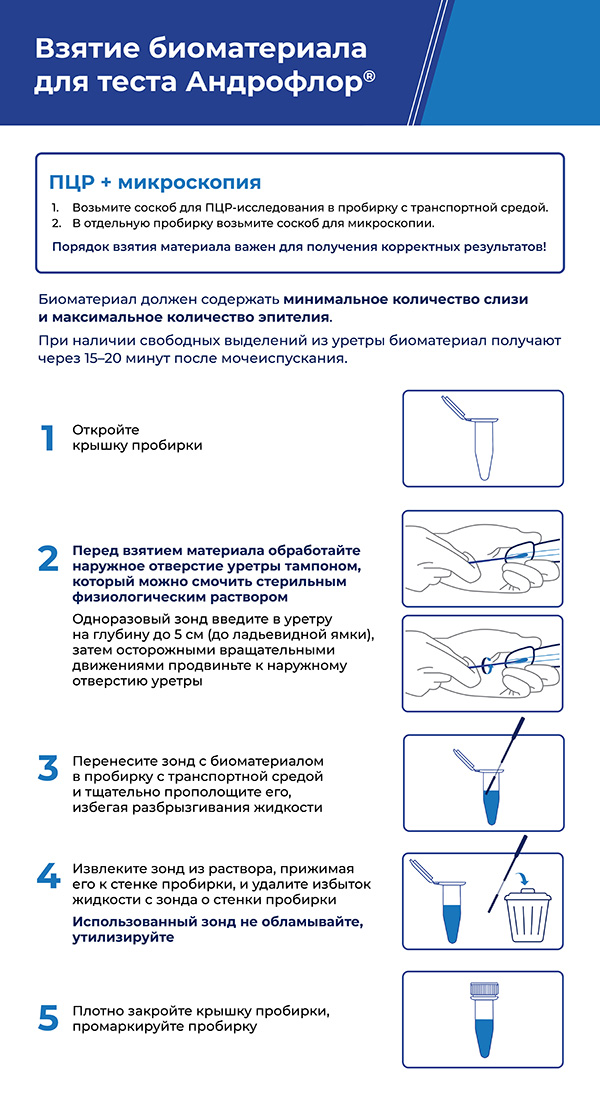
Standard pre-analytical recommendations are also relevant for Androflor®. Like any other infection diagnostics method (microscopy, microbiology, etc.), real-time PCR requires observation of rules for patient preparation and biomaterial collection and storage. Those rules include abstaining from sexual intercourses or using barrier contraception methods for at least 3 days (to minimize the risk of biomaterial contamination with partner’s microflora), excluding antibiotics or antiseptics (including antibacterial soap), abstaining from urination for 3 hours before scrape collection, recommendations to urinate and perform genital toilet before collection of prostate fluid or ejaculate, etc.
The most convenient biomaterial for the patient is ejaculate, which can be collected at home by masturbation. It is forbidden to collect ejaculate obtained by coitus interruptus (biomaterial will contain a major part of transient microflora) or ejaculate from a condom (lubricant components inhibit PCR). Collected biomaterial must be stored in a fridge for up to 24 hours or frozen. Strict biological sample storage conditions (urgent delivery to the laboratory, storage at a specific temperature) are not required, as the testing is performed using a non-cultivation-based method.

-
How long does the assay take?
Hands-on time from biomaterial acceptance in the laboratory is 4–6 hours. Please note that some time may be required for sample transportation, assay quality check, and in rare cases – for repeated test. The factor of laboratory workflow organization is also important. Therefore, the average turnaround time for the test is 1-2 days.
-
Why are there so many targets in the test? Why not reduce it to traditional STI screening?It is now obvious that the previously widespread approach of searching for specific pathogenic microbes in the reproductive tract is only informative for diagnosing STIs. In most cases, the source of unpleasant symptoms and patient complaints regarding urogenital infections is not the presence of pathogens (Chlamydia trachomatis, Mycoplasma genitalium, Neisseria gonorrhoeae, Trichomonas vaginalis), but rather a dysbiotic process – a disruption in the qualitative and quantitative balance of normal and opportunistic flora. To address this issue, real-time PCR can be used, as it simultaneously allows for the detection of major STIs in the biological sample and characterizes the contribution of various microbial groups to the total bacterial mass. The innovation that has made such diagnostics financially accessible for routine practice lies in the group identification of microorganisms and the application of multiplex analysis.
-
Is assigning several methods simultaneously recommended for diagnostics of infections?In case of infection testing, it is allowed to assign Androflor® simultaneously with microbiological assay and obtain the result for all the necessary groups of bacteria, including non-culturable anaerobic bacteria, in 1–2 days. It is also possible to carry out PCR tests for viruses (HSV, CMV, EBV, HPV) with the same biomaterial, at the discretion of physician.
-
Why do culture and Androflor® results not match?
Periodic discrepancies between culture results and Androflor® are due to fundamental differences between the two technologies. Culture involves cultivation followed by identification and assessment of antibiotic resistance for all microorganisms that have "grown." The results of culture directly depend on the ability of microorganisms to be cultured under the conditions provided by a specific bacteriological laboratory.
Androflor® detects the quantitative ratios of microorganisms directly based on DNA fragments that entered the tube during sample collection, regardless of the functional properties of the microorganisms.
Nevertheless, we can observe a correlation between culture and real-time PCR results, particularly in the detection of microorganisms from the Enterobacteriaceae family.
-
Is examining both sexual partners recommended in case of chronic infectious inflammatory disease?In these cases, it is recommended to examine both partners, using Femoflor® for women and Androflor® for men. Both tests are developed using the same real-time PCR technology. We have researched that microbiota of sexual partners can differ significantly. Therefore, it is crucial to screen the couple rather than prescribe empirical and often ineffective treatment for the woman based solely on her partner's test results, or vice versa (with the exception of STIs). Examining both sexual partners will help establish the etiology of the condition and select individualized therapy.
-
Is Androflor® consistent with current global trends?
Yes, in recent years, a global trend has emerged towards using high-tech methods to identify the microbiota of various human biotopes. International literature increasingly reports on the potential benefits of molecular genetic tests for diagnosing infectious-inflammatory processes of the genitourinary system. The primary method used is high-throughput DNA and RNA sequencing (Next Generation Sequencing, NGS), which is more suitable for research than routine practice due to the complexity and high cost of the technology.
Leading experts recognize the limitations of bacteriological research methods and associate prospects for improving diagnosis, and consequently treatment, with the application of nucleic acid analysis methods. These methods enable the selection of antimicrobial drugs targeting specific bacterial groups that caused the inflammatory response. This task becomes particularly relevant given the problem of antibiotic-resistant bacterial strains, which have emerged in response to the unsystematic use of broad-spectrum antibiotics. However, abroad in urology, molecular biological methods currently remain largely within the realm of scientific research.
During the development of the Androflor® test, which took over 7 years, DNA-Technology also used sequencing methods to create a prototype test with a large number of detectable parameters (over 50). After conducting scientific research on various sample groups, the diagnostic profile was adjusted, and an algorithm for interpreting results was developed. Androflor® currently has no foreign analogues, which is why the test is generating significant interest worldwide.
Quality guarantee
Androflor®, developed by Russian physicians and scientists, had been in use since 2016, with quality and security proved by clinical trials. Production facilities of DNA-Technology are equipped with modern instruments and tools. The production meets the requirements of GMP and ISO international quality standards, with each stage being controlled at the level of pharmaceutical production.

Androflor® is actively used in Russia and abroad for solving issues associated with etiological diagnostics of reproductively significant infections. In recent years, using high-end microbiota assessment methods has become a global trend.
FIND OUT MOREInformation
Ask questions about Androflor®
Please note that the specialists of the DNA Technology company provide consultations exclusively to medical specialists on the application and research features. Requests related to the appointment, delivery, or interpretation of tests are not considered. For relevant information, we recommend contacting the laboratory directly.